Impact of Electrodeposition on Tin Whisker Growth in Electronics
Introduction
This paper reviews two separate studies that investigate the electrodeposition of metals, specifically tin and tin-lead alloys, and their impact on the growth of tin whiskers in electronic devices.
Study 1
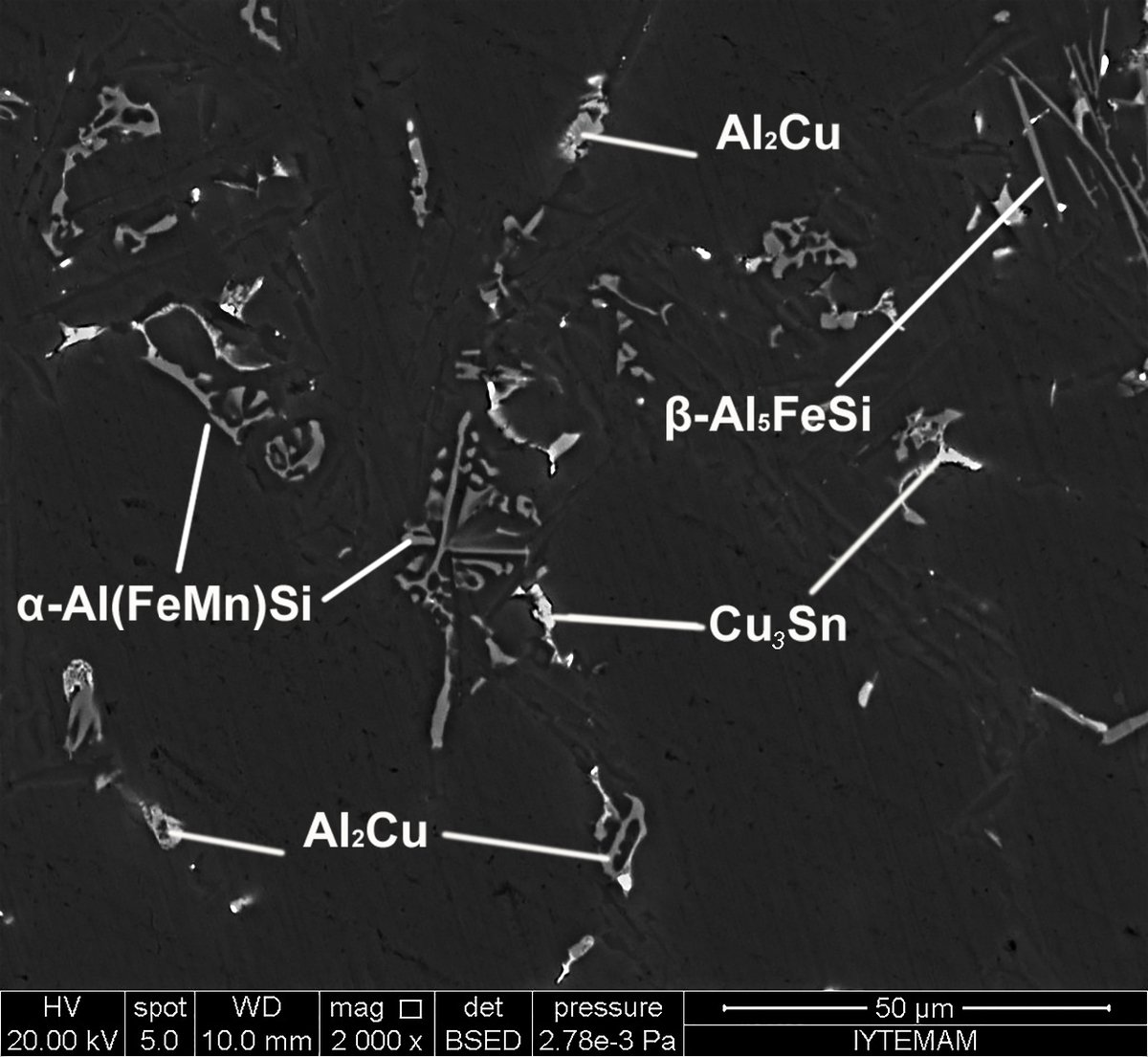
The first study aimed to understand the effect of adding lead to tin deposits on the growth of tin whiskers. Tin whiskers are thin protrusions that can cause short circuits and other issues in electronic devices. The study found that increasing the Pb content in the deposits alters the way copper diffuses into Sn grains, reducing the stress that could lead to whisker growth. This suggests that the intermolecular interaction between additives and surfactants can significantly affect electrodeposition processes and provide insight into the design of electrolytes for improved electrodeposition processes.
Study 2
A second study focused on the role of the intermolecular interaction between phthalic acid and non-ionic surfactant on the electrodeposition of metals. The study found that the chemical combination leads to the formation of a compact adlayer on the electrode surface, which inhibits the reduction reaction of metal ions on the cathode. The hydrophobic nature of these chemicals containing aromatic rings enhances the binding interaction and contributes to the blocking effect, which is governed by the intermolecular interaction. The study provides insight into the design of electrolytes for improved electrodeposition processes.
Methodology
Both studies analyzed matte tin electrodeposition at various Sn-Pb alloy compositions and evaluated several properties of the deposits, including crystallographic and microstructural characteristics, cathodic polarization, and the formation of Cu-Sn intermetallic compounds at the interface between the Sn or Sn/Pb films and the Cu substrate. The SEM image indicates that the deposit obtained from the electrolyte is smooth, regular, and compact. The low carbon content of the deposit suggests that the incorporation of the additive is strongly controlled in the presence of phthalic acid and non-ionic surfactant.
Conclusion
Overall, these studies provide valuable insights into the electrodeposition of metals and their applications in electronic devices. The findings can inform the design and manufacturing of electronic components, leading to more robust and reliable devices. The studies highlight the importance of intermolecular interactions between additives and surfactants in controlling the electrodeposition of metals and suggest strategies for preventing tin whisker formation in electronic devices. By understanding the mechanisms behind whisker growth, researchers and manufacturers can work towards developing more reliable electronic devices.
Note: These studies were not conducted by Advint Incorporated. Readers are required to do their own due diligence or contact Advint staff for proper technical guidance. Only use insights written by Venkat raja to gain technical knowledge.
You may like also

Posted By:Venkat Raja
Mar 17, 2023
Tags: