Mastering the Molecular Ballet: How PEG and PPG Revolutionize Copper Electrodeposition in Cutting-Edge Electronics
In the fast-paced world of modern electronics, the ability to create intricate circuitry on an increasingly miniature scale is paramount. At the heart of this technological marvel lies a process known as copper electrodeposition – a sophisticated technique that forms the backbone of printed circuit board (PCB) manufacturing and advanced microelectronics. But what truly elevates this process from mere metal plating to a precise science is the delicate dance of molecular additives, with polyethylene glycol (PEG) and polypropylene glycol (PPG) taking center stage as crucial suppressor molecules.
The Molecular Choreography of Copper Electrodeposition
Copper electrodeposition might sound straightforward – the process of depositing copper onto a surface using electricity – but its intricacies are far more complex. The real magic happens at the nanoscale, where PEG and PPG molecules interact with the copper surface in a carefully orchestrated performance that controls the rate and quality of copper deposition.
PEG: The Prima Ballerina of Suppressors
Polyethylene glycol, or PEG, is a hydrophilic polymer that plays a starring role in this molecular ballet. Its performance, however, is heavily dependent on a supporting cast – chloride ions. The relationship between PEG and chloride is so crucial that without chloride, PEG simply refuses to take the stage (or in scientific terms, adsorb to the copper surface).
When chloride ions are present, they facilitate a remarkable transformation. PEG molecules form a complex bridge structure on the copper surface, known as the PEG-Cu(I)-Cl− bridge. This intricate formation sees PEG’s oxygen atoms complexing with Cu(I) ions, which are in turn stabilized by the adsorbed chloride. The result? A robust suppression layer that acts as a barrier, controlling the reduction of copper ions at the electrode surface with exquisite precision.
PPG: The Contrasting Performer
Enter polypropylene glycol, or PPG – a more hydrophobic counterpart to PEG. PPG’s performance is distinctly different, adding depth and nuance to the molecular show. Its adsorption kinetics are slower, and it reaches surface saturation at a reduced rate compared to its PEG counterpart. Intriguingly, PPG causes a more negative suppression potential, hinting at a unique mechanism of action that complements PEG’s performance.
The Chloride Ion: More Than Just a Supporting Actor
The role of chloride ions in this molecular performance cannot be overstated. Far from being mere spectators, chloride ions are essential facilitators that influence every aspect of the suppressor molecules’ behavior:
- They are crucial for PEG adsorption, creating favorable conditions for these molecules to attach to the surface.
- Chloride ions help form the stable PEG-Cu(I)-Cl- complex, a key element in the suppression mechanism.
- They stabilize Cu(I) ions on the surface, promoting interaction with PEG’s oxygen atoms.
- Chloride ions occupy high-energy adsorption sites, complementing PEG’s preference for lower-energy sites and contributing to a more effective suppression layer.
- They significantly influence the adsorption-desorption dynamics of PEG, shifting the equilibrium strongly towards the adsorbed state.
This chloride-induced shift in equilibrium is not merely a kinetic effect but a fundamental change in the thermodynamic stability of adsorbed PEG. The result is enhanced surface coverage, conformational changes in PEG molecules (notably an increase in gauche conformation of C-O bonds), and the formation of a more stable and effective suppressor layer.
Spectroscopic Insights: Peeking Behind the Curtain
To truly appreciate the complexity of this molecular performance, researchers have employed advanced spectroscopic techniques, offering unprecedented insights into the behavior of these suppressor molecules:
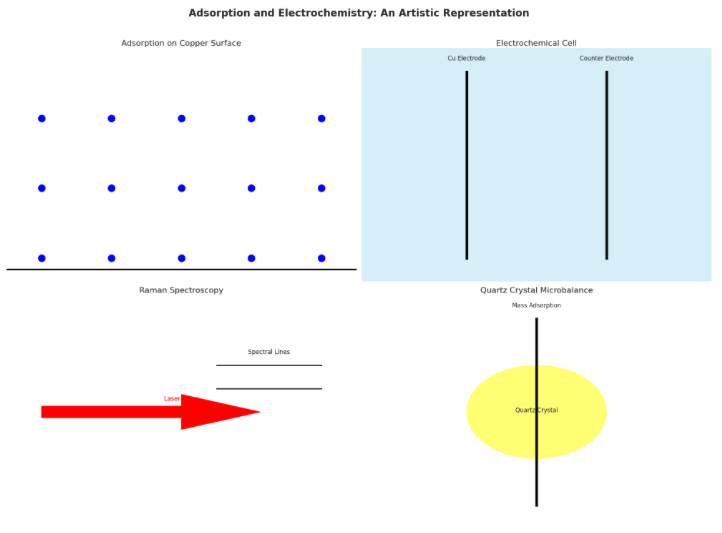
Raman Spectroscopy:
Both normal and surface-enhanced Raman spectroscopy (SERS) have revealed significant spectral shifts in PEG’s C-H stretching and bending regions upon surface adsorption. These shifts indicate conformational changes as PEG molecules adapt to their new role on the copper surface. PPG, true to its distinct character, shows less pronounced spectral changes, reflecting its reduced conformational flexibility.
Electrochemical Quartz Crystal Microbalance (QCM):
This sophisticated gravimetric technique has allowed researchers to quantify the adsorption process with remarkable precision. QCM studies have confirmed that PEG adsorption occurs only in the presence of chloride ions, while PPG can adsorb with or without chloride. Fascinatingly, PPG forms a denser surface layer (0.598 μg/cm²) compared to PEG (0.336 μg/cm²), highlighting how molecular structure influences adsorption behavior.
The Gauche Effect: A Twist in the Tale
Computational studies have added another layer of understanding to this molecular narrative. The observed spectroscopic trends are associated with an increased gauche character in the polymer backbone upon adsorption. This conformational change is not merely a curiosity but plays a pivotal role in the suppression mechanism, influencing the packing density and stability of the adsorbed layer.
Implications for Electrodeposition Kinetics
The distinct behaviors of PEG and PPG have profound implications for copper electrodeposition:
- Suppression Strength: PEG typically exhibits stronger suppression due to its more stable adsorption layer, while PPG’s weaker suppression may allow for more dynamic control of deposition rates.
- Desorption Kinetics: PPG is more readily desorbed by anti-suppressors like bis(3-sulfopropyl) disulfide (SPS), potentially allowing for more rapid modulation of local deposition rates.
- Fill Performance: The differences in adsorption behavior can be exploited to optimize the filling of high-aspect-ratio features, such as trenches and vias, in damascene processes crucial for advanced microchip fabrication.
Challenges and Future Directions
While PEG and PPG have revolutionized copper electrodeposition, challenges remain. The stability of these suppressor molecules under operating conditions is a key concern. Studies have shown that PEG-PPG copolymers can undergo degradation during the electrodeposition process, potentially impacting the consistency and quality of the deposited copper film over time.
Looking to the future, researchers are focusing on several promising avenues:
- Developing novel suppressor molecules with tailored hydrophobicity and molecular structures to further fine-tune the deposition process.
- Implementing in-situ spectroscopic monitoring for real-time process control, allowing for unprecedented precision in electrodeposition.
- Advancing molecular modeling techniques to predict suppressor behavior and guide additive design, potentially leading to breakthroughs in suppressor technology.
Conclusion: The Future of Molecular Engineering in Electronics
As we continue to push the boundaries of electronic miniaturization and performance, the insights gained from studying these suppressor molecules will be invaluable. The intricate dance of PEG, PPG, and chloride ions on copper surfaces is more than just a fascinating scientific phenomenon – it’s the key to unlocking the next generation of microelectronics.
Read More
Hydrogen Embrittlement: The Hidden Danger Compromising Metal Strength
By mastering this molecular ballet, we’re not just improving a manufacturing process; we’re paving the way for smaller, faster, and more efficient electronic devices that will shape our technological future.
From smartphones to supercomputers, the invisible performance of these molecular actors plays a crucial role in the devices we rely on every day.
As research in this field progresses, we can expect to see even more sophisticated control over the copper electrodeposition process, leading to advancements in electronics that we can scarcely imagine today. The molecular dance of PEG and PPG is just the beginning – a prelude to a future where molecular engineering drives the next great leaps in electronic technology.

Posted By:Venkat Raja
Jul 15, 2024
Tags: