Managing time with a data-driven approach offers incredible value in process management and customer service on electroplating and anodizing applications. Analytical data, awareness of chemicals and deposit properties with a historical context add value to engineers and aspiring scientists in the metal finishing field.
Spot and quick tests offer incredible insights for the metal finishing processes. What is a spot test? Are these tests reliable?
High temperature oxidation resistance is a valuable elemental and deposit property. What does it mean? Which elements possess this property? We will review the value of this physical property.
Surface and metal finishing offer a variety of options such as plating, anodizing, coloring of metals and electropolishing. Plating on several substrates such as plastic, steel, stainless steel, Invar, Kovar, nickel, aluminum, and titanium alloys are common. Elements like gold, silver, nickel, chromium, zinc and cadmium are plated. We choose the deposit based on consideration for cost, corrosion resistance and tribology properties.
Other than plating, we can electropolish and anodize metals such as stainless alloys. Applicators anodize on aluminum, magnesium, titanium, niobium, tantalum, etc.
Anodising is an electrolytic process in which we make an Al alloy anodic with a metal cathode on an acidic electrolyte. There are three classes of coating. We anodize with and without sealing.
This short paper explains which element to choose as deposit or substrate. It lists spot and quick tests available in the market. Review brightener chemistry and history. Discuss inhibitors, chemicals used for coloring metals and chromium conversion coatings.
Spot Tests
Several wet and instrumental analyses methods are used to conduct qualitative and quantitative analysis of elements. The advantage of these methods are accuracy and data reliability. But these test methods are time consuming and some are expensive. When you need a quick feedback, consider conducting spot tests on deposits and effluents. The sensitivity of spot test reaction can be at ppm levels. Elements such as aluminum, stannous tin, gold, silver, iron, nickel, palladium, lead, zinc, cadmium, chromium and copper can be tested. Many of these tests can take less than 5 minutes.
We can detect heavy metals, hexavalent ions and cyanide content in the effluent. You can distinguish between cadmium or zinc plating deposits using spot tests.
Here is a list of some organic chemicals used for spot tests:
Diphenyl carbazone
Dimethyl glyoxime
Tri-ammonium aurine-tricarboxylate
Nitro-bruciquinone hydrate
p-Dimethylamino benzylidene rhodamine
diphenyl thiocarbozone
sym-Diphenyl-carbazide
((1-2-Hydroxy-5-sulpho-phenyl)-3 phenyl-5-(2-carboxy-phenyl)-formzan) sodium salt
1-(2-Pydridyl-azo)-2-naphthol
Refer to Chem Spider for further information on any chemicals.
For further information on spot tests, read the book – Analysis of Metal Finishing Effluents and Effluent Treatment Solutions. This is a book written by Duncan MacArthur, Fred Stevens, and G. W. Fischer.
Chemicals
Did you know tobacco and licorice were one of the earlier brighteners used? Several decades ago, or even a century back, the use of brighteners or additives were very limited. Even the awareness of organic brightener science did not exit. Like many inventions, use of organic chemicals as a brightener was an accident. Tobacco was one of earlier recorded chemical used as additive. More than a century ago a plating operator who had the habit of chewing tobacco drooled the juice onto a plating solution during electroplating. Later noticed that a plated lot had a brighter appearance. Further investigation revealed the brightness influence of tobacco on plating deposit.
People used licorice during earlier days. It had a presence in the industry for some time and even now to an extent. Licorice extract is a carbohydrate and its chemical name is glycyrrhizin. They used it as an additive.
The additive was prepared by weighing a known quantity of licorice root and steep in a boiling water until colour saturation occurred. For 100 L plating solution, 100 grams of root was steeped in 0.5 L of boiling water.
Pickling and acid activation are a very common process at a steel mill, metal foundry, and a metal finishing processing plant. Conditional on activation and deposition bonding requirements, a substrate such as copper alloys or steel alloys would require activation under strongly acidic conditions. Use of strong acids such as hydrochloric acid, sulfuric acid and hydrofluoric acid can etch the substrates.
We commonly use sodium fluoride as an inhibitor. On picking applications, industry used antimony trioxide as an inhibitor.
Copper alloys such as brass tarnish in the presence of oxygen from atmosphere. Brass plated deposit do the same. An organic coating can prevent tarnish. Benzotriazole coating forms a thin layer in an immersion process, and the layer protects copper alloys from tarnish.
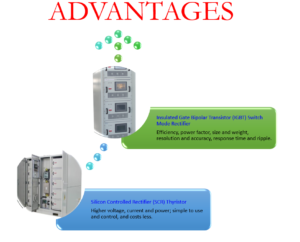
Brass plating is an alloy deposition process. The electromotive force potential of copper and zinc makes cyanide brass plating one of the most complex electrolytic processes. Attainment of a consistent and durable colour is tricky. Proper use of current density with an excellent choice of rectifier (IGBT or SCR) with an organic coating ensures great cosmetic appeal with durability.
We can also colour brass. One such colour is blue. An immersion process at high temperature in the presence of sodium sulphite and lead acetate colours brass substrates. Other than brass, we can colour stainless steel alloys using dichromate salts.
Yellow chromate on zinc deposit is one of the popular choices. We recognize the yellow chromate for brilliance of colour and corrosion protection. There is a subtle colour difference different between hexavalent and trivalent chromates. Chromates sometimes leaves iridescent finish. A protective coating similar to benzotriazole layer reduces iridescent streaks. One can get a reddish tone on the yellow chromate formulation. Use of a sulfate ions and nitric acid offers a reddish yellow chromate finish.
Occasionally people refer to chromate or chromium plating as chroming. Chroming is a colloquial speech term and we prefer you avoid using informal terms to avoid confusion and for use of clear language communication. Hexavalent Cr and (cyanide) cadmium is listed by Registration Evaluation Authorization and Restriction of Chemicals (REACH). We now replace cadmium plating with zinc / nickel alloy. Sacrificial protection of cadmium under saline conditions are inimitable.
Deposit Properties
Observing electromotive force series of elements and their potentials (negative or positive / active or noble) suggests evidences on fascinating deposit, material or electrolyte properties. We are referring to properties such as high temperature oxidation resistance and conductivity.
Pilling and Bedworth conducted a seminal work on high temperature oxidatio
Chromium, tantalum, zirconium and gold possess exceptional high temperature oxidation resistance properties. What is high temperature oxidation resistance?
The volume of oxide is greater or lesser than the parent metal, it produces or cannot produce an effective protective property.
What does a reference to protective property mean? It refers to the formation of an oxide layer, such as tantalum oxide, chromium oxide and zirconium oxide on the metal or the deposit. The refractory metals offer oxidation resistance up to ~ 600ºF. Oxidation and healing property are the principal reason hexavalent hard chromium plated components had gained wide popularity. Many applications of aerospace and automotive industries require components to posses tribological properties at a higher temperature. Wear, lubrication, and friction are such examples. The oxides can re-form and withstand high temperatures during these mechanical transformations. Tantalum, zirconium, niobium, and chromium metals are a few among the best to possess such a property. Other than oxidation properties, these elements are susceptible to corrosion resistance from acids, alkalis, organic media and other reagents. We measure high temperature oxidation in ratio and Pilling – Bedworth (PB) ratio of corrosion resistant metals range between 0 and 4. Higher the number better the corrosion resistance. PB ratio is the ratio of the metal oxide volume divided by the metal volume. Chromium PB ratio is 2. All chromium electroplating deposits do not have the same corrosion resistance properties. Corrosion resistance of decorative Cr plating is because of nickel undercoat. Most hard chromium deposit do not have any corrosion resistance. However, a few formulations containing fluoride ions in the electrolyte possess high corrosion resistance. Some hard-hexavalent Cr deposits pass 500 hours of neutral salt spray test (NSST). We mainly attribute the variations to formulation, processing, and process control.
Other than refractory metals, precious metals such as gold plating deposit possess high temperature oxidation resistance. Industry uses gold plated components on space applications because of high temperature oxidation resistance, conductivity, high surface stability, high resistance to tarnish, and chemical corrosion. Both trivalent and monovalent salts are used to deposit gold from electrolytes. Precious metals like gold, silver and palladium can be plated on several substrates such as stainless steel, Kovar, Inconel, magnesium, aluminum and titanium alloys efficaciously.
Besides lightweight, the stubborn oxide layer makes aluminum and titanium alloys indispensable in our daily lives.
Previous paragraph mentioned about the conductivity of deposit. What about electrolyte conductivity? Change of ion activities with concentration affects electrolyte conductivity. Understanding interionic attraction theory of electrolytes are essential to improve conductivity.
Value
Whether you are an engineer or a research scientist, understanding element and deposit properties, electrolyte capabilities and limitations, and vitality of unique chemicals are important. A process design engineer with a good understanding on these characteristics can design products with superior corrosion and tribological properties. The matters covered in this paper such as high temperature oxidation resistance can help a designer identify suitable metal as substrate and a deposit.
We identify some chemicals listed in this paper as hazardous or carcinogen per Registration Evaluation Authorization and Restriction of Chemicals (REACH). REACH is a European Union regulation. You can also find additional information on carcinogens by visiting the website of National Institute of Environmental Health Sciences (NIEHS) under the National Toxicology Program (NTP). Observing regulatory compliance and identifying risk mitigation plan will drive an organization’s governance.
REACH, quality demand, and customer requirement will call for a transformed focus on a few facets such as colouring of metals, plating solution additives, chromium conversion coating and anodizing. An ardent electroplating specialist must consider all the services, test methods and fundamental concepts.
A leader must prepare electroplating companies to manage complex processes. An agile metal finishing organization aiming to survive even under adverse conditions shall be data driven, ensure speed of business is appreciable, work faster, and inculcate easy-to-use test methods. We know many that plating companies who are not data driven do not grow or adapt to developing changes.
Spot testing of effluent, electrolyte or deposit is an under used method, and will help gather data with speed and ease. Other quick tests like pH measurement, specific gravity, litmus, refractometer, and profilometer are all easy and inexpensive. This do not mean volumetric and instrumental analysis offer less value. Both methods are vital for many electroplating operations. The choice depends on their technical ability, product testing requirements and financial capability. The examples of these instruments are atomic absorption spectroscopy, scanning electron microscopy, induced couple plasma (ICP), x-ray diffraction, x-ray fluorescence and electron microprobe analysis. We use these units for elemental analysis at lower concentration with a top-level accuracy. XRD and EPMA can detect light elements such as lithium, oxygen and carbon with an outstanding repeatability and reproducibility.
We deliberated chemicals, methods, properties, and data driven approach. Cognizance of these matters without a long-term analysis and reaction plan is not noteworthy! Consider use of run chart, control chart and Process Development and Control (PDC) tools with a visual dashboard.
Readers can find a value on other short papers written on this page previously. Please read articles on electrode potential, IGBT and SCR power supplies, current distribution, throwing power, periodic table, Time Change Management (TCM), Process Development & Control (PDC) tools, and communication. A complex electrolysis process requires a multidimensional approach on disciplines such as science, mathematics, technology and management. There are many vital aspects involved in this field such as automation, process control, business development methodologies, and so on. Fundamentals, laws, equations, and concepts govern electrolysis. Though not needed on a day-to-day basis, these are important to be aware and apply. Discipline, observation, data, patterns, and behaviors are critical for one to succeed at a higher level. Though there will be a scientific explanation for all outputs, one needs to treat the work as art! This is imperative because of our limitations – time and knowledge. Hence, at Advint we offer on guidance on subjects related to laboratory practices, equipment engineering, automation, productivity, lean, statistical process control (SPC) tools, and management. Advint’s virtual Electroplating Training explains all these subjects comprehensively.
You may like also
Electronics Manufacturing through Reel to Reel Plating | Advint Incorporated