Acknowledgement of the influential in electroplating develops our learning of the fundamentals, and it improves our research.
There are thousands of contributors who served the electroplating industry and academia for over a century.
Who tops your list?
What did they do?
Why is it important?
What did we learn?
his short paper lists 8 innovators, two focused on electrochemistry and the rest on industrial plating (electrolytic, autocatalytic, ionic & aqueous). Their contributions enabled our understanding, improved our applications, and helped us to advance the technology.
Let us see who they are, when and what did they offer to the field and its benefits.
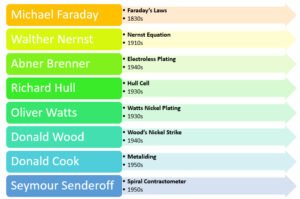
Michael Faraday
During 1832 Faraday published a paper relating the quantity of electricity with the amount of metal liberated at the electrodes resulting in two laws. When we think of electroplating, Faraday’s laws are probably the first to come to our mind. Mastering a process or shining at customer satisfaction does not end without due consideration of an electrolyte’s current efficiency and the feasibility of a deposit.
Walther Nernst
Nernst equation is the fundamental equation of electrochemistry and for the electrode processes. An ardent electroplating researcher shall begin and ensure a thorough understanding of Nernst equation and its concept. We can plate several transition metals, post transition metals, and metalloids. Some using aqueous electrolytes and room temperature ionic liquids, and most using high temperature ionic liquids. A modest approach to choose the electrolyte and the element for the deposition process is to comprehend Nernst equation and its concept. An interstitial or interatomic alloy electrodeposit choice is no exception to this subject.
Abner Brenner
Brenner conducted many studies on electroplating deposits. We conspicuously recognize for his preliminary contributions on electroless nickel plating invention around 1946. Though he got black non-adherent dendrites in the initial testing, his research allowed significant growth in electroless Ni-P and Ni-B deposition processes, and plating on plastics.
Richard Hull
Hull cell device is common in most electroplating laboratories. Most platers gain Hull cell testing skills or aspire to become good at it! Hull around 1930s invented a testing cell and derived a formula to calculate the effective current density of an electrolyte. Hull cell unit and its formula is quite easy to use! Is the design of the unit and its formula an ordinary achievement?
Oliver Watts
Professor Watts reported on 1931 his work on nickel plating bath comprising nickel sulfate, nickel chloride and boric acid at a higher temperature. Many used nickel-plating electrolyte using the same inorganic constituents at room temperature or at 120ºF. Watts was the first to report the benefits of thick and uniform deposit at 145ºF and 160ºF. Since then we began calling this electrolyte a Watts nickel plating solution.
Donald Wood
Wood was an expert in cyanide silver plating, and that is what he did for most of his career. We know him for his invention of chloride-based nickel strike bath during early 1940s. The Wood’s nickel strike formulation enabled the global industry to plate on all ferrous, nickel, titanium and aluminum alloys.
Donald Cook
Even if you are well read in the industry, you might not have heard about Dr. Donald Cook! Cook coined the term ‘metaliding’. Metal finishing industry did not see the effects of his research. But if I didn’t mention his name with others, it would be amiss as he is alike Nernst and Brenner. No one would have worked on more transition elements and diffused into others than him! His knowledge in electrochemistry and chemistry of halides was impeccable, and he distinguished the ins and outs of transition metals of group 3 to 11 of the periodic table. But his only focus was in high temperature ionic liquids.
Seymour Senderoff
We know Dr. Senderoff for his invention of spiral contractometer used in hard hexavalent chromium and nickel sulfamate plating applications to detect internal stress in the deposit. He worked for Dr. Brenner for several years and later focused on high temperature ionic liquid tantalum plating. It was my source of pride to continue on his research and improvise his formulation.
So, what is the learning?
Recognizing these pioneers and their work must transcend awareness and dwell on a deeper understanding of their explanations and research outputs.
Faraday and Nernst focussed on the fundamental of electrochemistry. Cook and Senderoff concentrated mostly on high temperature ionic liquid electrolysis (plating and diffusion). Brenner, Hull, Watts and Wood dedicated their research on commercial electrolysis. Nevertheless, all played a revolutionary role in electroplating applications.
Here are the examples of teachings from a few of these pioneers’ work:
Nernst’s invention allowed us to relate electrode potential, valency of the metal ion and current. Hull’s work brought to light Tafel’s, Butler’s and Volmer’s work. Wood’s invention signified electromotive force (emf) series and distinguished strike, flash plating, and plating. Cook’s and Senderoff’s developments emphasized the importance of eutectic temperature, phase diagram, liquidus temperature of salts, fluxing effect and ionic conductivity of electrolytes.
Hope you find this paper valuable and you dig deeper on these matters!
Post your comments and write about who tops your list.
You may like also